Systematic Review Wit Hmeta Anlysis on Obesity and Periodontal Disease
- Research article
- Open Admission
- Published:
Influence of obesity on the effect of non-surgical periodontal therapy - a systematic review
BMC Oral Health volume 16, Article number:90 (2016) Cite this article
Abstract
Groundwork
Obesity and periodontitis are of import chronic wellness problems. Obesity is associated with an increased prevalence of periodontitis. Whether obesity also affects the result of not-surgical periodontal therapy is to engagement nevertheless unclear.
Methods
A systematic review of studies referenced in SCOPUS, MEDLINE, PubMed, Cochrane, CINAHL, Biosis and Web of Scientific discipline was performed. Titles, abstracts and finally full texts were scrutinized for possible inclusion by ii independent investigators. Quality and heterogeneity of the studies were assessed and the study designs were examined. Probing pocket depth reduction was analyzed as primary surrogate parameter for therapeutic success after not-surgical periodontal therapy.
Results
One-hundred-and-fifty-nine potentially qualifying studies were screened. Eight studies fulfilled the inclusion criteria and were analyzed. 3 of viii studies failed to evidence an influence of obesity on pocket depth reduction after non-surgical therapy. The remaining five studies documented a clear negative event on the consequence of non-surgical periodontal therapy. The finally included studies did not correspond to the highest level of quality (RCTs). Due to the heterogeneity of the data a meta-analysis was not possible.
Conclusion
The literature on the effect of obesity on the treatment effect of non-surgical periodontal therapy remains controversial. The information, nonetheless, support that obesity is non only a factor associated with poorer periodontal health but might besides result in inferior response to non-surgical treatment of periodontitis.
Groundwork
The prevalence of obesity is increasing worldwide and is becoming one of the almost important health hazards [1], every bit obesity is highly associated with increased overall morbidity and bloodshed [ii].
Obesity is divers with a trunk mass index (BMI; body weight in kilogram divided past the foursquare of the tiptop in meters (kg/m2)) of at to the lowest degree 30.0 kg/m2 [3], whereas overweight is defined with a BMI of 25–29.ix kg/mii. Normal weight is characterized by a BMI ranging between 19 to 24.ix kg/1000ii [4]. In this context, BMI seems a valuable parameter to predict obesity-related illness risks in a wide range of populations [2]. In that location are, notwithstanding, some limitations: Firstly, risk assessment past BMI is less applicable in persons over 65 years of age because they generally have a college torso fat content for the aforementioned BMI. Secondly, the abdominal (fundamental, visceral, android) type of obesity, which is more than often seen in men, is associated with higher morbidity than the rather female person type of gluteofemoral (peripheral, gynoid) obesity and, thirdly, the BMI cut-off points for overweight and obesity are too high for Asian people [2]. In addition, current large studies have indicated that measurement of waist circumference (WC) or waist-to-hip-ratio (WHR) may be a better disease risk predictor than BMI [5, six]. There is, notwithstanding, currently intensive inquiry and debate every bit to whether BMI, WC, WHR, or all of them should be used to assess disease hazard [two].
For the purpose of this systematic review, however, but BMI is the most frequently reported information of obesity in a large number of studies.
Adipose tissue contains usually 5-10 % macrophages, but the adipose tissue of obese patients shows up to 60 % macrophage infiltration [iv]. Adipocytes secrete bioactive molecules called adipokines, that can modify or trigger inflammation and fat metabolism locally or systemically as signaling molecules to liver, muscle and endothelium [4]. Therefore, the adipose tissue can be considered as an important metabolically agile endocrine organ [four].
This explains how obesity acts every bit a chance factor for several chronic diseases: Hypertension, type 2 diabetes, dyslipidemia, and coronary centre disease are so closely related to obesity that obesity itself is often considered to exist a systemic affliction. This disease also affects dental wellness [7]. Accordingly obese persons require attention of physicians and dentists [2].
Amid dental pathologies, periodontitis is a very mutual, primarily bacterial inflammatory disease, which destroys teeth surrounding soft tissues and bone. Information technology leads to pocket germination and ultimately to loss of teeth if no constructive treatment is applied [eight]. Periodontitis is no longer considered only an oral wellness event only as well a public health problem, equally it constitutes a risk cistron for cardiovascular conditions, poor glycemic control in diabetics and agin effect of pregnancy [4, 8]. These correlations coincide with obesity and general health.
Recently, information technology has been suggested that obesity is a possible risk factor for periodontitis [eight]. One report identified obesity even every bit the second strongest risk factor for periodontitis preceded only past smoking [9]. The first written report on the human relationship between obesity and periodontal disease appeared in 1977. Perlstein and co-workers [10] institute greater alveolar bone resorption in obese than in non-obese rats. Under salubrious oral conditions, obesity itself did non promote periodontal damage, but in the presence of bacterial plaque accumulation periodontal inflammation was more severe in obese than in non-obese animals. With concomitant arterial hypertension, plaque accumulation caused fifty-fifty more than pronounced periodontal destruction than with obesity alone. These results advise that a combination of risk factors, such as the i divers by the metabolic syndrome, elicit a more astringent periodontal event [10, 11]. Chaffee et al. [12] found in their meta-analysis an increased prevalence odds ratio for obesity amongst subjects with periodontal disease of approximately i-tertiary, a greater hateful clinical attachment loss (CAL) among obese individuals, a higher BMI among subjects with periodontal affliction, and a trend for linear increment in the odds of periodontal illness with increasing BMI [4, 12]. Finally the association reported betwixt obesity and periodontitis was less potent than that reported between periodontal disease and agin pregnancy outcomes [12, 13] or cardiovascular events [12, 14]. There seems, still, to be a stronger obesity-periodontitis association in women, non-smokers and younger individuals than in the general adult populations [12]. In add-on, smoking remains some other well-studied predisposing cistron for periodontitis [12, 15, 16]. Thus, BMI and smoking share a complex human relationship [17]. This relationship tin can be inverse in certain populations [12, 18, nineteen].
The biological mechanism by which obesity predisposes to periodontitis is not fully understood [8]. Compared to individuals with normal weight individuals with obesity have college levels of circulating tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-half dozen), which are also secreted from adipose tissue and are involved in the pathophysiology of both obesity and periodontitis. Non surprisingly, serum levels of these cytokines subtract with loss of weight [xx].
The objective of this systematic review was to study the hypothesis whether the clinical issue, in terms of pocket depth reduction, subsequently non-surgical periodontal therapy in not-obese is amend than in obese individuals. To verify this hypothesis, we systematically reviewed all retrievable, qualitatively adequate clinical investigations, which focused on this topic.
Methods
The review was conducted according to the PRISMA criteria [21]. The research question was explored using the PICO method [22). The focused question addressed was:
Does non-surgical periodontal therapy (I) have a different outcome in obese chronic periodontitis patients (P), than in not-obese chronic periodontitis patients (C), regarding periodontal pocket depth reduction as the chief clinical periodontal parameter (O).
Search strategy and review process
An electronic search of SCOPUS, MEDLINE, PubMed, Cochrane, CINAHL, Biosis and Web of Science was carried out considering articles published up to January 2016 in English language or German linguistic communication. The search was performed in two steps. The first electronic search started at 20.xi.14 and an update has been washed at v.ane.16.
This is shown in Table i. For the database search, a combination of subject headings (MeSH terms and CINAHL headings) and costless text search was used. An example of a detailed strategy (Medline/OvidSP) is shown in Table 2.
The same search protocol was practical to all databases.
Two of the authors (FAG, PRS) screened the titles for potential eligibility co-ordinate to the inclusion criteria. Based on the abstract screening, 18 studies were selected for total text review. Scores were independently allocated past both authors to each publication according to their suitability for the present review (see inclusion criteria). Whatsoever discrepancies were resolved by consensus.
Inclusion criteria
To be included studies had to be clinical interventional studies regarding the outcome of non-surgical periodontal therapy in obese or non-obese patients. The studies had to display the diagnosis of chronic periodontitis. Key parameters to be reported were data for pocket probing depth (PPD) and BMI.
Exclusion criteria
Studies were excluded for the following reasons: animal studies, case reports, commentaries, unsuitable exposure or effect measures, confounding medical diagnoses (e.g. pregnancy or any systemic disease, such every bit diabetes, in add-on to metabolic syndrome), misreckoning systemic medical treatments such every bit immunosuppressive treatments, cortisone or antibody treatment as well as confounding local treatments such as treatment of peri-mucositis, gingival overgrowth or surgical periodontal handling. Studies including either the diagnosis of aggressive periodontitis or of peri-implantitis were excluded as well.
Consequence measures
The primary outcome measure is PPD after non-surgical periodontal treatment.
Data extraction
A list with exclusion reasons for each newspaper was generated. Total number of patients, demographic data, origin of written report, outcome measurements two, 3, 6 and 12 months after therapy and the impact of obesity on the treatment-outcome were extracted. In addition, the exact definition of chronic periodontitis, the assessment of the periodontal illness and the number of smokers included in the studies were summarized. Data on the individual definition of obesity and the systemic examinations were also nerveless. Non-surgical periodontal treatment measures, treatment time, periodontal maintenance and agin events were also recorded for each written report separately (Tables iii, 4, 5, six and vii).
The quality of the included studies was assessed through the Newcastle-Ottawa Quality Cess Scale (Tabular array 8).
Results
Choice of studies
Initially, 159 studies were identified by electronic search by the ii reviewers (FAG, PRS). Full text analysis of the 18 potentially qualified reports led to exclusion of ten other studies. Additional five titles [23–27] were identified by hand search but after full text analysis, all these articles had to exist excluded based on the inclusion and exclusion criteria (Tabular array iii). Therefore, 8 publications [8, 28–34] from the electronic and mitt search fulfilled the criteria. Notwithstanding, it was not possible to compare the raw data and so that we had to reduce our assay to a qualitative analysis. This process is summarized in a flow-chart (Fig. 1).
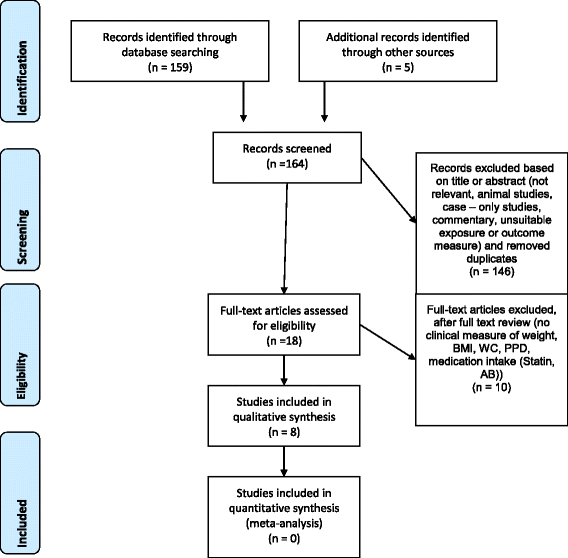
PRISMA 2009 flow diagram
Summary of studies: characteristics (PICO)
Total number of patients, demographic information, origin of study, outcome measurements 2, iii, 6 and 12 months later on therapy and the touch of obesity on the handling-outcome are summarized in Table 4.
Definition of chronic periodontitis, periodontal assessment and the amount of smokers included are depicted in Table 5.
The definition of obesity and systemic examination were summarized in Tabular array six. Non-surgical periodontal treatment, treatment fourth dimension, periodontal maintenance and limitations are shown in Tabular array vii.
Population
Of the eight finally analyzed studies, clinical trials comprised 516 participants. One study [viii] enrolled only women. The prevalence of smoking among male person patients affected with periodontitis was so high that Al-Zahrani and co-workers [eight] were unable to correct for smoking in the male population. Accordingly, only women were included in their study. Overall the studies comprised betwixt 26 upwards to 260 subjects (Tabular array 4).
The inclusion criteria "historic period" was divers in all but one study [30]. 3 investigations [28, 33, 34] defined the age ≥xviii years. The other studies defined a minimum historic period of thirty years [31, 32] or 35 years [viii]. Duzagac et al. divers an age range from 25 to 55 years (Table 4).
Suvan et al. [34] included smokers into their written report. Lakkis et al. [33] and Eldin et al.(30] did not mention the smoking condition of the patients. All other studies [8, 28, 29, 31, 32] excluded smokers (Table 5).
Patients with diabetes, another important modifier of periodontal health or disease [35] were excluded in 7 studies [8, 28–32, 34]. Lakkis et al. [33] did not report the presence or absence of diabetes.
Intervention/Comparison
Each paper described the periodontal intervention as a not–surgical therapy. All studies [8, 28–34] practical scaling and root planing. Ultrasonic instruments and/or hand instruments were used in all studies (Table 7).
2 papers (8,34] reassessed the PPD 2 months after therapy. Two studies [29, 30] reassessed their patients after iii months. Another two papers [28, 31] re-evaluated the patients later on three and half dozen months and ane study [32] reassessed the PPD three, six and 12 months after therapy. Just Lakkis et al. [33] measured the periodontal pocket depths already iv to 6 weeks later on non-surgical therapy (Tabular array 3). Oral hygiene was instructed additionally to the non–surgical periodontal therapy in all studies (Tabular array seven).
Due to the heterogeneity of the written report designs with respect to issue measures and treatment protocol, every bit well as variation in study population, sample size, and/or statistical methods, a statistical synthesis of the results of the included studies was non possible. And so the authors decided to analyze the papers on a qualitative way [22]. A meta-assay was not possible.
Outcome
Generally obese patients were found to have deeper periodontal pockets at baseline in all studies.
Iii [8, 29, 30] of the eight papers [8, 28–34] reported no major negative bear upon of obesity on response to periodontal therapy in terms of PPD reduction (mm). Al-Zahrani et al. [8] assessed the reduction of PPD (mm) comparison obese with normal–weighted women. At that place was no statistically significant event of obesity on handling outcome. Duzagac et al. [29] assessed the clinical response to non-surgical periodontal treatment, according to the severity of periodontitis based on probing depth < 4 mm vs. ≥ iv mm. Patients with and without obesity showed similar clinical healing in terms of per centum and number of sites with probing depth < 4 mm and ≥ 4 mm. So they failed to show an effect of obesity on the treatment outcome dependent on the severity of the affliction. Eldin et al. [thirty] likewise institute no result when comparison an overweight group with an obese group. The departure between the groups in reduction of PPD was not significant (Table four).
Five [28, 31–34] of the eight papers showed a negative result of obesity on the healing afterward not-surgical periodontal therapy. Bouaziz et al. [28] revealed that normal-weight patients had a better response to periodontal treatment than obese patients. This upshot was specially observed for moderate-to-deep pockets. This fact suggests that the more than severe the periodontitis the more pronounced is the negative consequence of obesity on periodontal treatment outcome. They showed in the multivariate assay that obesity was significantly associated with percentage changes of PD > 5 mm and numbers of improving sites (p ≤ 0.05). In the univariate analysis all periodontal parameters improve more in patients suffering from more astringent periodontitis at baseline. Other patient characteristics, like age, sexual practice, obesity, and WHR, were not associated with periodontal parameter changes. Gonçalves et al. [31] showed that patients with obesity and chronic periodontitis had a lower PDD reduction than patients without obesity. The measurement of the reduction in PPD (mm) at full-mouth sites showed later 3 months a non statistically significant difference (p = 0.08) between the obese group compared to the group without obesity. However after 6 months there was a statistically meaning difference (p = 0.04). At this time point, especially deep sites (PPD ≥ 7 mm) showed a significantly better result in the group without obesity (p = 0.04). Another study of Gonçalves et al. [32] reported that patients with obesity had a significantly greater mean PD (half dozen months p-value = 0.04, 12 months p value = 0.03) than patients without obesity at six and 12 months postal service-therapy. The data of a written report past Suvan et al. [34] corroborated these findings and showed that obesity was an contained impact value of poorer periodontal treatment outcome 2 months after therapy. The extent of the clan between poorer periodontal treatment and obesity was like to that of smoking (p = 0.02). They worked with 260 patients. This secondary analysis consisted of individuals participating in five clinical studies of non-surgical periodontal therapy over a 7-year menstruum. Lakkis et al. [33] selected thirty patients who were obese; fifteen of them had previously undergone bariatric surgery, whereas the other half (n = fifteen) did not loose any weight and served as a control group. The bariatric surgery group reached a statistically pregnant greater mean PPD reduction (0.45 mm versus 0.28 mm) compared with the control (no surgery) group (p = 0.007; Table 4).
Limitations
Some patients could not be reviewed or discontinued the study for personal reasons [28, 29, 31]. Weight loss and pregnancy were additional reasons to exist excluded in the written report of Duzagac et al. [29] (Table vii).
The quality of the included studies was evaluated through the Newcastle-Ottawa Quality Assessment Calibration (Table viii).
Discussion
This review focused on obesity and the outcome after not-surgical periodontal therapy and has shown that currently there is no really robust scientific evidence to reach solid conclusions and recommendations.
Three papers [8, 29, 30] were institute, which did not find whatever statistically significant negative bear on of obesity on the response to not-surgical periodontal therapy, whereas five papers [28, 31–34] showed the opposite, i.eastward. a conspicuously negative influence of obesity on the treatment outcomes.
With regard to the quality of prove, seven included papers [8, 28, 29, 31–34] reported some limitations.
Al-Zahrani et al. [8] included only women. Gonçalves and co-workers [31] did non consistently apply the accepted definitions of overweight and obesity, but rather included the waist-to-hip-ratio (WHR) for their definitions, probably leading to inclusion of patients with a BMI inferior to xxx kg/mtwo into the obesity grouping. Thus the results are hard to interpret and compare with the other studies and are therefore not applicable for patients with a BMI ≥forty kg/thousand2 [31]. All the same, these studies still show a conspicuously ameliorate tendency with regard to the treatment response for patients without obesity every bit divers in their written report. Lakkis et al. [33] chose another interesting manner to find an impact of obesity on the outcome of non-surgical periodontal treatment. They compared obese people who had undergone bariatric surgery (BS) with obese who did not. Afterward weight loss in the BS grouping, a reduction in total adipocytes might have resulted in a decrease in adipokines and pro-inflammatory mediators released by those adipose cells. This systemic inflammatory reduction might take played a role in reducing the insulin resistance resulting in a improve outcome after periodontal therapy every bit suggested by the authors [33]. Some limitations in the specific contour of the obese patients (nondiabetic, non-smoker) in the paper of Bouaziz et al. (28) may restrict the extrapolation of the results to the whole obese population. Furthermore, the small sample size may too limit the power of this study. Duzagac et al. [29] failed to include a control group of periodontally good for you controls with obesity. Additionally the hateful periodontitis parameters were inside the limits of "moderate" periodontitis, and the WHR and BMI values of these obese patients were predominantly below those characterizing morbid obesity. So, the results of this written report may not be extrapolated to those with astringent periodontitis or morbid obesity. The 2nd included study of Gonçalves and co-workers [32] assumes that the high inter-patient variance in adipokine levels may reduce the statistical power to detect handling effects, equally previously reported. The results presented by Suvan et al. [34] may take been influenced by study limitations linked with unequal numbers in BMI categories and sample size. In addition, at that place may take been limitations with regard to the interpretation associated with the post hoc secondary analysis experimental design, although variation in clinical assessment and treatment was minimized past examiner and treatment clinician stability. This study did not plant a higher level of evidence in the context of testify-based wellness care levels of scientific show [34].
Overall, obesity is an obvious, visible stigma and so that the studies cannot be considered blinded. This may be another possible bias in each of the studies.
Five studies [viii, 28, 29, 31, 32] excluded smokers. Since smoking influences periodontal health these studies are biased [ix] and may non exist fully representative for the typical overall population. Overall it appears, nevertheless, that a positive event of normal weight is present in not-smokers [28, 31, 32] and in smokers [34].
Equally mentioned before, the included studies differed in statistical methods, populations, sample sizes, definition of chronic periodontitis, definition of obesity, fourth dimension of upshot measurement, smoking status, periodontal assessment and not-surgical periodontal therapy. Therefore, it was not only impossible to perform a meta-assay just also draw articulate conclusions.
Nonetheless at that place is a consensus in the studies that obesity is associated with different baseline PPD levels. The large cohort in the written report of Suvan et al. [34] and the long term results of Gonçalves et al. [31, 32] may lead to the conclusion that obesity is an important negative factor which influences non-surgical periodontal therapy.
In summary, all studies [viii, 28, 29, 31–34] included in this review validated the efficacy of non-surgical periodontal therapy, except the study of Eldin and co-workers [30] who did not study any efficacy of the therapy (Table iii). Clinically, it appears obvious that a therapy is necessary to reach periodontal health independent of the patient's body mass index.
Considering this systematic review provided only moderate evidence that obesity is an important factor for not-surgical periodontal therapy, future prospective cohort studies are needed to confirm these findings [36]. Such trials should exist of loftier methodological quality. They should control important confounding factors such as smoking condition, severity of chronic periodontitis, severity of obesity. Every patient should become the aforementioned periodontal treatment and periodontal maintenance. Overall, there is a possibility to solve the research question even though blinding of the examiners to obesity or non-obesity status is non practically possible.
Clinicians should know that obesity may accept some influence on periodontal status and are likely to have a negative touch on on the clinical outcome of conservative treatment, even if this systematic review found only five [28, 31–34] out of 8 papers [viii, 28–34] corroborating the influence of obesity on the clinical periodontal effect focusing on PPD as surrogate parameter for periodontal healing.
Clinicians might consider a weight reduction diet every bit an boosted handling for periodontal health with a positive consequence expected after vi and 12 months [31, 32]. Also it should not be neglected that weight control has substantial other beneficial health effects which on their ain justify such a recommendation.
Conclusion
This systematic review indicates a possible negative human relationship between obesity and poorer treatment event in obese patients afterwards not-surgical therapy based on the results of five out of eight studies. Three of these studies denied an impact of obesity on the treatment. The potentially junior healing response could be based on pathophysiological inflammatory models.
Baseline levels showed also a poorer periodontal health in patients with obesity compared with not-obese patients.
No study found any better dental health parameters in obese than in non-obese individuals, and although dental wellness may not be the most of import target for arriving at a about normal body weight, a person who tin keep his or her body weight nigh normal might, in addition to all other established health benefits, count on having meliorate periodontal health than if they are obese.
References
-
Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper torso obesity and periodontitis. J Dent Res. 2001;eighty:1631–6.
-
Pischon North, Heng North, Bernimoulin JP, Kleber BM, Willich SN, Pischon T. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86:400–nine.
-
Francesco Branca HNuTL. Dice Herausforderung Adipositas und Strategien zu ihrer Bekämpfung in der Europäischen Region der WHO. 2007.
-
Suresh South, Mahendra J. Multifactorial relationship of obesity and periodontal disease. J Clin Diagn Res. 2014;8:ZE01–03.
-
Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of intestinal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–63.
-
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–nine.
-
Słotwińska Southward. Host response, obesity, and oral health. Cent Eur J Immunol. 2015;40:201–5.
-
Al-Zahrani MS, Alghamdi HS. Effect of periodontal treatment on serum C- reactive protein level in obese and normal-weight women affected with chronic periodontitis. Saudi Med J. 2012;33:309–14.
-
Nishida NTM, Hayashi Northward, Nagata H, Takeshita T, Nakayama Thou, et al. Determination of smoking and obesity as periodontitis risks using the classification and regression tree method. J Periodontol. 2005;76:923–8.
-
Perlstein MI, Bissada NF. Influence of obesity and hypertension on the severity of periodontitis in rats. Oral Surg Oral Med Oral Pathol. 1977;43:707–19.
-
Koletsky Due south. Obese Spontaneously Hypertensive Rats-A Model for Study of Atherosclerosis. Exp Mol Pathol. 1973;nineteen:53–60.
-
Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81:1708–24.
-
Wimmer GPB. A critical assessment of agin pregnancy outcome and periodontal disease. J Clin Periodontol. 2008;35:380–97.
-
Blaizot A, Vergnes JN, Nuwwareh S, Amar J, Sixou One thousand. Periodontal diseases and cardiovascular events: metaanalysis of observational studies. Int Dent J. 2009;59:197–209.
-
Tomar SL, Asma South. Smoking-attributable periodontitis in the United States: findings from NHANES Three. National Health and Diet Examination Survey. 2000;71:743–51.
-
Tonetti MS. Cigarette smoking and periodontal diseases: etiology and direction of affliction. Ann Periodontol. 1998;1:88–101.
-
Arnaud Chiolero DF, Fred P, Jacques C. Consequences of smoking for body weight, trunk fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–9.
-
Fei Xu, BEcon, Xiao-Mei Yin BM, Youfa Wang. The association between amount of cigarettes smoked and overweight, central obesity amidst Chinese adults in Nanjing, China. Asia Pac J Clin Nutr. 2007;16:240-247.
-
Hou 10, Jia W, Bao Y, Lu H, Jiang S, Gu H, Xiang K. Run a risk factors for overweight and obesity, and changes in body mass index of Chinese adults in Shanghai. BMC Public Health. 2008;8:389.
-
Altay U, Gurgan CA, Agbaht Chiliad. Changes in inflammatory and metabolic parameters later periodontal handling in patients with and without obesity. J Periodontol. 2013;84:13–23.
-
David Moher AL, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol. 2009;62:1006–12.
-
Buset SL, Zitzmann NU, Weiger R, Walter C. Non-surgical periodontal therapy supplemented with systemically administered azithromycin: a systematic review of RCTs. Clin Oral Investig. 2015;19(8):1763–75.
-
D'Aiuto F, Nibali L, Parkar K, Suvan J, Tonetti MS. Curt-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269–73.
-
Duan JY, O-YX, Zhou YX. Result of periodontal initial therapy on the serum level of lipid in the patients with both periodontitis and hyperlipidemia. Bejing Da Xue Xue Bao. 2009;41:36-39.
-
Iwamoto Y, Nishimura F, Soga Y, Takeuchi K, Kurihara M, Takashiba S, et al. Antimicrobial periodontal treatment decreases serum C-reactive poly peptide, tumor necrosis factor-alpha, but not adiponectin levels in patients with chronic periodontitis. J Periodontol. 2003;74:1231–vi.
-
Kardeşler BN, Cetinkalp S, Kinane DF. Adipokines and inflammatory mediators after initial periodontal treatment in patients with blazon 2 diabetes and chronic periodontitis. J Periodontol. 2010;81:24–33.
-
Tandon S, Lamba AK, Verma Yard, Munjal A, Faraz F. Effect of Periodontal Therapy on Serum Lipid Levels. Indian J Med. 2010;1:19–25.
-
Bouaziz West, Davideau JL, Tenenbaum H, Huck O. Adiposity Measurements and Non-Surgical Periodontal Therapy Outcomes. J Periodontol. 2015;86:1030–7.
-
Duzagac E, Cifcibasi E, Erdem MG, Karabey Five, Kasali K, Badur S, et al. Is obesity associated with healing afterwards not-surgical periodontal therapy? A local vs. systemic evaluation. J Periodontal Res. 2015. [Epub ahead of print]
-
Eldin AM, Nasr SA, Hassan NE. Issue of non-surgical periodontal therapy on interleukin-eight(il-eight) level in gingival crevicular fluid in overweight and obese subjects with chronic periodontitis. Earth J Med Sci. 2013;ix:173–9.
-
Goncalves TE, Feres G, Zimmermann GS, Faveri Grand, Figueiredo LC, Braga PG, et al. Furnishings of scaling and root planing on clinical response and serum levels of adipocytokines in patients with obesity and chronic periodontitis. J Periodontol. 2015;86:53–61.
-
Goncalves TE, Zimmermann GS, Figueiredo LC, Souza Mde C, da Cruz DF, Bastos MF, et al. Local and serum levels of adipokines in patients with obesity subsequently periodontal therapy: one-year follow-up. J Clin Periodontol. 2015;42:431–9.
-
Lakkis D, Bissada NF, Saber A, Khaitan Fifty, Palomo L, Narendran S, et al. Response to periodontal therapy in patients who had weight loss after bariatric surgery and obese counterparts: a pilot report. J Periodontol. 2012;83:684–9.
-
Suvan J, Petrie A, Moles DR, Nibali L, Patel K, Darbar U, et al. Body mass index every bit a predictive factor of periodontal therapy outcomes. J Dent Res. 2014;93:49–54.
-
Genco RJ, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–84.
-
Sgolastra F, Severino Yard, Pietropaoli D, Gatto R, Monaco A. Effectiveness of Periodontal Treatment to Improve Metabolic Control in Patients With Chronic Periodontitis and Blazon 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials. J Periodontol. 2013;84:958–73.
-
Europerio 4, Berlin, Deutschland, 19-21 June 2003. J Clin Periodontol. 2003;xxx:5-100.
-
Abstracts for the Imperial Australian and New Zealand College of Psychiatrists 40th Congress. Psychiatry in a Changing World, Sydney, Australia, Sunday 22-Thursday 26 May, 2005. Aust N Z J Psychiatry. 2005;39:A97–186.
-
fifth Joint Meeting of the European Tissue Repair Club and the Wound Healing Society. Wound Repair Regen. 2009;17:A54–87.
-
Recently published abstracts. Altern Med Rev. 2010;fifteen:369–eighty.
-
DENTSPLY. Posters. J Dent Hyg. 2012;86:322–6.
-
HealthBeat. Dent Assist. 2007;76:59–63. 55p.
-
Abstracts for Poster Presentations. second Northward American/ Global Dental Hygiene Inquiry Conference Bethesda, MD, October xx-22, 2011. J Dent Hyg. 2012;86:39–53. 15p.
-
Abstracts for the International Symposium on Dental Hygiene, Greatcoat Town, South Africa, August xiv-17, 2013. Int J Dent Hyg. 2013;11:156–73.
-
Abou Sulaiman AE, Shehadeh RMH. Assessment of Total Antioxidant Capacity and the Use of Vitamin C in the Handling of Non-Smokers With Chronic Periodontitis. J Periodontol. 2010;81:1547–54.
-
Acharya A, Bhavsar N, Jadav B, Parikh H. Cardioprotective effect of periodontal therapy in metabolic syndrome: a pilot study in Indian subjects. Metab. 2010;8:335–41.
-
Akpinar A, Toker H, Ozdemir H, Bostanci V, Aydin H. The furnishings of not-surgical periodontal therapy on oxidant and anti-oxidant status in smokers with chronic periodontitis. Arch Oral Biol. 2013;58:717–23.
-
Armstrong BL, Sensat ML, Stoltenberg JL. Halitosis: a review of electric current literature. J Paring Hyg. 2010;84:65–74.
-
Arora N, Avula H, Avula JK. The adjunctive use of systemic antioxidant therapy (lycopene) in nonsurgical handling of chronic periodontitis: A short-term evaluation. Quintessence Int. 2013;44:399–409.
-
Basegmez C, Berber L, Yalcin F. Clinical and Biochemical Efficacy of Minocycline in Nonsurgical Periodontal Therapy: A Randomized Controlled Pilot Study. J Clin Pharmacol. 2011;51:915–22.
-
Bresolin AC, Pronsatti MM, Pasqualotto LN, Nassar PO, Jorge AS, da Silva EAA, et al. Lipid profiles and inflammatory markers after periodontal treatment in children with congenital heart affliction and at risk for atherosclerosis. Vasc Health Hazard Manage. 2013;9:703–9.
-
Bresolin AC, Pronsatti MM, Pasqualotto LN, Nassar PO, Jorge AS, Da Silva EAA, et al. Effectiveness of periodontal treatment on the comeback of inflammatory markers in children. Curvation Oral Biol. 2014;59:639–44.
-
Caspersen CJ, Thomas GD, Boseman LA, Beckles GLA, Albright AL. Crumbling, Diabetes, and the Public Wellness System in the United States. Am J Public Health. 2012;102:1482–97.
-
Caula AL, Lira-Inferior R, Tinoco EM, Fischer RG. The consequence of periodontal therapy on cardiovascular chance markers: a 6-month randomized clinical trial. J Clin Periodontol. 2014;41:875–82.
-
Chapple ILC. Oxidative stress, nutrition and neutrogenomics in periodontal health and disease. Int J Dent Hyg. 2006;iv:15–21.
-
Chapple ILC, Brock GR, Milward MR, Ling N, Matthews JB. Compromised GCF total antioxidant capacity in periodontitis: crusade or effect? J Clin Periodontol. 2007;34:103–10.
-
Chandni R, Mammen J, Joseraj MG, Joseph R. Effect of Nonsurgical Periodontal Therapy on Insulin Resistance in Patients with Type 2 Diabetes Mellitus and Chronic Periodontitis. Diabetes. 2015;64:A692.
-
Chaston R, Sabatini R, Koertge TE, Brooks CN, Schenkein HA. Serum anticardiolipin concentrations in patients with chronic periodontitis post-obit scaling and root planing. J Periodontol. 2014;85:683–7.
-
Chee HK, Lim LP, Tay F, Thai Air conditioning, Sum CF. Non-surgical periodontal handling and lipid levels in diabetic patients. Ann R Australas Coll Dent Surg. 2008;19:183.
-
Chee B, Park B, Bartold P. Periodontitis and blazon II diabetes: a two-way relationship. Int J Evid-Based Healthcare. 2013;eleven:317–29. 313p.
-
Chen L, Luo Thousand, Xuan D, Wei B, Liu F, Li J, et al. Furnishings of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: a randomized report. J Periodontol. 2012;83:435–43.
-
D'Aiuto F, Parkar One thousand, Andreou G, Brett PM, Ready D, Tonetti MS. Periodontitis and atherogenesis: causal association or simple coincidence? A pilot intervention written report. J Clin Periodontol. 2004;31:402–xi.
-
D'Aiuto F, Set D, Tonetti MS. Periodontal disease and C-reactive protein-associated cardiovascular risk. J Periodontal Res. 2004;39:236–41.
-
D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Middle J. 2006;151:977–84.
-
Deppe H, Hohlweg-Majert B, Holzle F, Schneider KT, Wagenpfeil S. Pilot study for periodontal treatment and pregnancy effect: a clinical prospective written report. Quintessence Int. 2010;41:e101–110.
-
Dodington DW, Fritz PC, Sullivan PJ, Ward Nosotros. Higher Intakes of Fruits and Vegetables, beta-Carotene, Vitamin C, alpha-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers simply Not in Smokers. J Nutr. 2015;145:2512–9.
-
Draper C. Advances in applied science and periodontal therapy. J Calif Dent Hyg Assoc. 2010;25:12–iv.
-
Edwards J. Exploring the link between oral health & systemic disease. Access. 2006;20:15–20.
-
Efurd MG, Bray KK, Mitchell Telly, Williams K. Comparing the Risk Identification and Management Behaviors betwixt Oral Health Providers for Patients with Diabetes. J Dent Hyg. 2012;86:130–40.
-
Elliott-Smith Southward. Periodontal Illness: A Patient-by-Patient Approach. Access. 2011;25:28–32.
-
Engebretson SP, Hyman LG, Michalowicz BS. Hemoglobin a1c levels among patients with diabetes receiving nonsurgical periodontal handling - Reply. JAMA. 2014;311:1921–ii.
-
Fairfield C. Photodisinfection -- innovative adjuntive therapy. J Calif Paring Hyg Assoc. 2010;25:20–two.
-
Fang F, Wu B, Qu Q, Gao J, Yan Due west, Huang X, et al. The clinical response and systemic furnishings of non-surgical periodontal therapy in terminate-stage renal disease patients: a half dozen-calendar month randomized controlled clinical trial. J Clin Periodontol. 2015;42:537–46.
-
Fentoglu O, Sozen T, Oz SG, Kale B, Sonmez Y, Tonguc MO, et al. Curt-term effects of periodontal therapy equally an adjunct to anti-lipemic treatment. Oral Dis. 2010;16:648–54.
-
Fine JB. The influence of periodontal inflammation on systemic diseases and medical conditions. Access. 2007;21:14–9.
-
Fokkema SJ. Peripheral blood monocyte responses in periodontitis. Int J Dent Hyg. 2012;10:229–35.
-
Fu YW, Li XX, Xu HZ, Gong YQ, Yang Y. Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: a randomized controlled trial. Clin Oral Investig. 2015;20:1263–9.
-
Garcia VG, Takano RY, Fernandes LA, de Almeida JM, Theodoro LH. Treatment of experimental periodontal disease past a selective inhibitor of cyclooxygenase-2 with scaling and root planing (SRP). Inflammopharmacology. 2010;18:293–301.
-
Giblin LJ, Boyd LD, Rainchuso L, Chadbourne D. Short-term effects of not-surgical periodontal therapy on clinical measures of impaired glucose tolerance in people with prediabetes and chronic periodontitis. J Dent Hyg. 2014;88 Suppl 1:23–30.
-
Gluch JI. Commentary on "The consequence of periodontal therapy on TNF-blastoff IL-half-dozen and metabolic control in type 2 diabetics". Access. 2007;21:26–viii.
-
Goldie MP. Perio trends. Dispelling myths about periodontal illness. Access. 2002;16:40.
-
Goldie MP. C-reactive protein, cardiovascular disease, and periodontal disease. Int J Dent Hyg. 2004;2:139–41.
-
Goldie MP. What is new in research? Antioxidants in oral health intendance: making the connexion. Int J Dent Hyg. 2005;three:93–5.
-
Griffin So, Jones JA, Brunson D, Griffin PM, Bailey WD. Burden of Oral Disease Among Older Adults and Implications for Public Health Priorities. Am J Public Wellness. 2012;102:411–viii.
-
Gurenlian JR. Inflammation: the relationship between oral health and systemic illness. Access. 2006;20:1–9. 9p.
-
Gurenlian JR. Inflammation: the relationship between oral health and systemic illness. Dent Assist. 2009;78:8.
-
Hammaker BG. Pharmacologic interventions for controlling the inflammatory cascade in periodontal illness. Admission. 2010;24:xix.
-
Horwitz J, Hirsh I, Machtei EE. Oral aspects of Gaucher's affliction: a literature review and instance written report. J Periodontol. 2007;78:783–8.
-
Hovliaras-Delozier CA. A common phonation. Access. 2008;22:22–9.
-
Ide M, Jagdev D, Coward PY, Cheat Grand, Barclay GR, Wilson RF. The short-term furnishings of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol. 2004;75:420–8.
-
Ide M. Intensive periodontal treatment including extractions is associated with more than immediate systemic inflammation but improved longer-term endothelial office compared to simple scaling. J Evid Based Paring Pract. 2007;7:162–four. 163p.
-
Jahn C. Diabetes and periodontal health. Dent Help. 2004;73:24–9.
-
Jahn C. STANDARDS. Standard one: Cess: III. Hazard Assessment. Admission. 2015;29:19–23. 15p.
-
Jaiswal GR, Jain VK, Dhodapkar SV, Kumathalli KI, Kumar R, Nemawat A, et al. Impact of bariatric surgery and diet modification on periodontal status: A six month cohort study. J Clin Diagn Res. 2015;9:ZC43–five.
-
Janket SJ. Scaling and root-planing (SRP) may ameliorate glycemic control and lipid contour in patients with chronic periodontitis (CP) and type 2 diabetes (DM2) in a specific subgroup: a meta-analysis of randomized clinical trials. J Evid Based Dent Pract. 2014;14:31–3.
-
Jared H, Boggess KA. Periodontal diseases and agin pregnancy outcomes: a review of the evidence and implications for clinical practise. J Dent Hyg. 2008;82:24–41.
-
Jiang H, Xiong Ten, Su Y, Zhang Y, Wu H, Jiang Z, et al. A randomized controlled trial of pre-conception handling for periodontal disease to amend periodontal status during pregnancy and birth outcomes. BMC Pregnancy Childbirth. 2013;xiii:228.
-
Kamil W, Al Habashneh R, Khader Y, Al Bayati L, Taani D. Effects of nonsurgical periodontal therapy on C-reactive poly peptide and serum lipids in Jordanian adults with advanced periodontitis. J Periodontal Res. 2011;46:616–21.
-
Kamilov KP. Human relationship between chronic periodontitis clinical manifestation and lipid peroxidation in saliva. Uzbekiston Tibbiet Zhurnali. 1998;0:37–9.
-
Kapellas K, Practise LG, Mark Bartold P, Skilton MR, Maple-Brown LJ, O'Dea K, et al. Effects of total-oral cavity scaling on the periodontal health of Indigenous Australians: a randomized controlled trial. J Clin Periodontol. 2013;40:1016–24.
-
Keller A, Rohde JF, Raymond 1000, Heitmann BL. Association between periodontal disease and overweight and obesity: a systematic review. J Periodontol. 2015;86:766–76.
-
Kiany F, Hedayati A. Evaluation of serum anti-cardiolipin antibodies after non-surgical periodontal treatment in chronic periodontitis patients.Odontology. 2014;103:203–9.
-
Kiany F, Hedayati A. Evaluation of serum anti-cardiolipin antibodies after not-surgical periodontal treatment in chronic periodontitis patients. Odontology. 2015;103:203–9.
-
Kipp A, Majeski J. New trends in perio. Access. 2003;17:ten–5.
-
Kudva P, Tabasum ST, Garg N. Evaluation of clinical and metabolic changes after not surgical periodontal handling of type ii diabetes mellitus patients: A clinico biochemical study. J Indian Soc Periodontol. 2010;xiv:257–62.
-
Kumar M, Bandyopadhyay P, Mishra L, Das Due south, Kundu PK, Mistry Due south. Result of periodontal therapy on glycemic command and circulating TNF-α in blazon two diabetic patients. Int J Diabetes Dev Ctries. 2015;35:96–102.
-
Kurti B, Tüter G, Serdar G, Pinar S, Demirel I, Toyman U. Gingival crevicular fluid prostaglandin E(ii) and thiobarbituric acid reactive substance levels in smokers and non-smokers with chronic periodontitis post-obit phase I periodontal therapy and adjunctive utilise of flurbiprofen. J Periodontol. 2007;78:104–111.
-
Kurtis B, Tuter Thousand, Serdar M, Pinar S, Demirel I, Toyman U. Gingival crevicular fluid prostaglandin Due east-two and thiobarbituric acrid reactive substance levels in smokers and not-smokers with chronic periodontitis following stage I periodontal therapy and adjunctive utilize of flurbiprofen. J Periodontol. 2007;78:104–xi.
-
Lee HJ, Jun JK, Lee SM, Ha JE, Paik DI, Bae KH. Association between obesity and periodontitis in meaning females. J Periodontol. 2014;85:e224–231.
-
Li X, Tse HF, Yiu KH, Zhang C, Jin LJ. Periodontal therapy decreases serum levels of adipocyte fat acid-binding protein in systemically healthy subjects: a pilot clinical trial. J Periodontal Res. 2013;48:308–xiv.
-
Ling A, Wang P-W, Lin R-T, Hsieh C-J, Lee P-Y, Zhuang R-Y, et al. Evaluation of Periodontal Status and Effectiveness of Not-Surgical Handling in Patients With Blazon ii Diabetes Mellitus in Taiwan for a ane-Year Menses. J Periodontol. 2012;83:621–8.
-
Lo JC, O'Ryan F, Yang J, Hararah MK, Gonzalez JR, Gordon Northward, et al. Oral Wellness Considerations in Older Women Receiving Oral Bisphosphonate Therapy. J Am Geriatr Soc. 2011;59:916–22. 917p.
-
Malhotra R, Kapoor A, Grover V, Grover D, Kaur A. Effect of Scaling and Root Planing on Erythrocyte Count, Hemoglobin and Hematocrit in Patients with Chronic Periodontal Disease. J Dent Hyg. 2012;86:195–203.
-
Mancl KA, Kirsner RS, Ajdic D. Wound biofilms: Lessons learned from oral biofilms. Wound Repair Regen. 2013;21:352–62.
-
Martinez GL, Koury JC, Brito F, Fischer RG, Gustafsson A, Figueredo CM. The impact of non-surgical periodontal handling on serum levels of long chain-polyunsaturated fatty acids: a pilot randomized clinical trial. J Periodontal Res. 2014;49:268–74.
-
Martinez GL, Koury JC, Martins MA, Nogueira F, Fischer RG, Gustafsson A, et al. Serum level changes of long concatenation-polyunsaturated fat acids in patients undergoing periodontal therapy combined with one yr of omega-3 supplementation: a pilot randomized clinical trial. J Periodontal Implant Sci. 2014;44:169–77.
-
Matlock J, Ferguson Thousand, Calef J, Abbott Southward. Obesity and Bariatric Surgery: Effects on the Oral Cavity. Access. 2012;26:viii–xi.
-
McDaniel JC, Roy Southward, Wilgus TA. Neutrophil activity in chronic venous leg ulcers-A target for therapy? Wound Repair Regen. 2013;21:339–51. 313p.
-
Meharwade VV, Gayathri GV, Mehta DS. Effects of scaling and root planing with or without a local drug delivery system on the gingival crevicular fluid leptin level in chronic periodontitis patients: a clinico-biochemical study. J Periodontal Implant Sci. 2014;44:118–25.
-
Merchant AT. Hemoglobin A1c levels amidst patients with diabetes receiving nonsurgical periodontal treatment [1]. JAMA. 2014;311:1919.
-
Michalowicz BS, Hyman L, Wei H, Oates Jr TW, Reddy Yard, Paquette DW, et al. Factors associated with the clinical response to nonsurgical periodontal therapy in people with type 2 diabetes mellitus. JADA. 2014;145:1227–39. 1213p.
-
Mizrak T, Guncu GN, Caglayan F, Balci TA, Aktar GS, Ipek F. Effect of a controlled-release chlorhexidine scrap on clinical and microbiological parameters and prostaglandin East-ii levels in gingival crevicular fluid. J Periodontol. 2006;77:437–43.
-
Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh Chiliad. Non-surgical periodontal therapy affects metabolic command in diabetics: a randomized controlled clinical trial. Aust Dent J. 2012;57:31–37.
-
Moravec LJ, Boyd LD. Bariatric Surgery and Implications for Oral Wellness: A Example Written report. J Dent Hyg. 2011;85:166–76.
-
Muthu J, Muthanandam Southward, Mahendra J, Namasivayam A, John L, Logaranjini A. Effect of Nonsurgical Periodontal Therapy on the Glycaemic Control of Nondiabetic Periodontitis Patients: A Clinical Biochemical Written report. Oral Wellness Prev Paring. 2015;xiii:261–6.
-
Nassar PO, Walker CS, Salvador CS, Felipetti FA, Orrico SRP, Nassar CA. Lipid profile of people with Diabetes mellitus type 2 and periodontal disease. Diabetes Res Clin Pract. 2012;96:35–9.
-
Newton KM, Chaudhari M, Barlow WE, Inge RE, Theis MK, Spangler LA, et al. A population-based study of periodontal care among those with and without diabetes. J Periodontol. 2011;82:1650–vi.
-
Nichols FC, Levinbook H, Shnaydman M, Goldschmidt J. Prostaglandin E2 secretion from gingival fibroblasts treated with interleukin-1beta: effects of lipid extracts from Porphyromonas gingivalis or calculus. J Periodontal Res. 2001;36:142–52.
-
Nielsen D, Walser C, Kodan G, Chaney RD, Yonkers T, VerSteeg JD, et al. Effects of treatment with clindamycin hydrochloride on progression of canine periodontal illness later ultrasonic scaling. Vet Ther. 2000;i:150–8.
-
Novakovic N, Cakic S, Todorovic T, Raicevic BA, Dozic I, Petrovic Five, et al. Antioxidative Status of Saliva before and after Non-Surgical Periodontal Handling. Srp Ark Celok Lek. 2013;141:163–eight.
-
Novakovic Northward, Todorovic T, Rakic M, Milinkovic I, Dozic I, Jankovic Due south, et al. Salivary antioxidants every bit periodontal biomarkers in evaluation of tissue condition and treatment outcome. J Periodontal Res. 2014;49:129–36.
-
Oliveira AM, de Oliveira PA, Cota LO, Magalhaes CS, Moreira AN, Costa FO. Periodontal therapy and gamble for adverse pregnancy outcomes. Clin Oral Investig. 2011;xv:609–xv.
-
Olsen NC. Health Access. 2006;twenty:56–7. 52p.
-
Paquette DW, Ryan ME, Wilder RS. Locally delivered antimicrobials: clinical bear witness and relevance. J Paring Hyg. 2008;82:ten–5.
-
Perayil J, Suresh Northward, Fenol A, Vyloppillil R, Bhaskar A, Menon S. Comparing of Hba1c Levels in Non-Diabetic Healthy Subjects and Subjects With Periodontitis Before and Later Non-Surgical Periodontal Therapy. J Periodontol. 2014:85:1658–66.
-
Perayil J, Suresh N, Fenol A, Vyloppillil R, Bhaskar A, Menon S. Comparing of glycated hemoglobin levels in individuals without diabetes and with and without periodontitis before and later non-surgical periodontal therapy. J Periodontol. 2014;85:1658–66.
-
Phillips A. Oral complications of diabetes: an under-recognized condition. Pract Nurs. 2013;24:562–six.
-
Pradeep AR, Manjunath SG, Swati PP, Shikha C, Sujatha Pb. Gingival crevicular fluid levels of leukotriene B4 in periodontal health and disease. J Periodontol. 2007;78:2325–30.
-
Price T. Periodontal disease and agin pregnancy outcomes: handling recommendations for the pregnant adult female. Access. 2010;24:twenty–3.
-
Qiqiang L, Huanxin Chiliad, Xuejun K. Longitudinal written report of volatile fatty acids in the gingival crevicular fluid of patients with periodontitis before and after nonsurgical therapy. J Periodontal Res. 2012;47:740–ix.
-
Radafshar Chiliad, Torabi F, Mirfarhadi N. Short-term effects of intensive non-surgical periodontal therapy and depression-dose doxycycline on serum levels of IL-6, TNF-blastoff and lipid profile in advanced periodontitis. Afr J Microbiol Res. 2012;six:355–60.
-
Radnai M, Pal A, Novak T, Urban E, Eller J, Gorzo I. Benefits of periodontal therapy when preterm nativity threatens. J Dent Res. 2009;88:280–four.
-
Raghavendra NM, Pradeep AR, Kathariya R, Sharma A, Rao NS, Naik SB. Effect of not surgical periodontal therapy on gingival crevicular fluid and serum visfatin concentration in periodontal health and disease. Dis Markers. 2012;32:383–8.
-
Ramirez JH, Arce RM, Contreras A. Periodontal handling effects on endothelial function and cardiovascular disease biomarkers in subjects with chronic periodontitis: protocol for a randomized clinical trial. Trials. 2011;12:46.
-
Rasch MS, Mealey BL, Prihoda TJ, Woodard DS, McManus LM. The effect of initial periodontal therapy on salivary platelet-activating factor levels in chronic adult periodontitis. J Periodontol. 1995;66:613–23.
-
Sadatmansouri S, Sedighpoor N, Aghaloo M. Effects of periodontal treatment phase I on nascence term and nativity weight. J Indian Soc Pedod Prev Dent. 2006;24:23–six.
-
Saffi MA, Furtado MV, Montenegro MM, Ribeiro IW, Kampits C, Rabelo-Silva ER, et al. The effect of periodontal therapy on C-reactive protein, endothelial office, lipids and proinflammatory biomarkers in patients with stable coronary artery disease: study protocol for a randomized controlled trial. Trials. 2013;fourteen:283.
-
Williams KB. Issue of treating periodontal disease on cardiovascular markers. J Dent Hyg. 2007;81:49.
-
Sembene M, Moreau JC, Mbaye MM, Diallo A, Diallo PD, Ngom K, et al. Periodontal infection in pregnant women and low birth weight babies. Odontostomatol Trop. 2000;23:19–22.
-
Sengupta S, Fine J, Wu-Wang CY, Gordon J, Murty VLN, Slomiany A, et al. The relationship of prostaglandins to camp, IgG, IgM and alpha-ii- macroglobulin in gingival crevicular fluid in chronic adult periodontitis. Arch Oral Biol. 1990;35:593–half-dozen.
-
Shimada Y, Komatsu Y, Ikezawa-Suzuki I, Tai H, Sugita Northward, Yoshie H. The event of periodontal handling on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010;81:1118–23.
-
Shimoe M, Yamamoto T, Iwamoto Y, Shiomi Northward, Maeda H, Nishimura F, et al. Chronic periodontitis with multiple risk factor syndrome: a case report. [Erratum appears in J Int Acad Periodontol. 2011 Oct;13(three):93]. J Int Acad Periodontol. 2011;13:40–vii.
-
Singh Northward, Narula SC, Sharma RK, Tewari Due south, Sehgal PK. Vitamin E Supplementation, Superoxide Dismutase Status, and Result of Scaling and Root Planing in Patients With Chronic Periodontitis: A Randomized Clinical Trial. J Periodontol. 2014;85:242–ix.
-
Siqueira MAD, Fischer RG, Pereira NR, Martins MA, Moss MB, Mendes-Ribeiro Air conditioning, et al. Furnishings of non-surgical periodontal treatment on the L-arginine-nitric oxide pathway and oxidative status in platelets. Exp Biol Med. 2013;238:713–22.
-
Stewart JE, Wager KA, Friedlander AH, Zadeh HH. The issue of periodontal handling on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol. 2001;28:306–ten.
-
Talbert J, Elter J, Jared HL, Offenbacher S, Southerland J, Wilder RS. The effect of periodontal therapy on TNF-alpha, IL-half-dozen and metabolic control in type ii diabetics. J Paring Hyg. 2006;80:vii–7.
-
Tamaki N, Tomofuji T, Ekuni D, Yamanaka R, Yamamoto T, Morita M. Short-Term Effects of Non-Surgical Periodontal Handling on Plasma Level of Reactive Oxygen Metabolites in Patients With Chronic Periodontitis. J Periodontol. 2009;80:901–6.
-
Tamaki Due north, Tomofuji T, Ekuni D, Yamanaka R, Morita Yard. Periodontal handling decreases plasma oxidized LDL level and oxidative stress. Clin Oral Investig. 2011;xv:953–viii.
-
Tawfig A. Effects of non-surgical periodontal therapy on serum lipids and C-reactive protein among hyperlipidemic patients with chronic periodontitis. J Int Soc Prev Community Dent. 2015;5:S49–56.
-
Teles FR, Teles RP, Martin L, Socransky SS, Haffajee Advertizing. Relationships among interleukin-half-dozen, tumor necrosis gene-alpha, adipokines, vitamin D, and chronic periodontitis. J Periodontol. 2012;83:1183–91.
-
Toker H, Akpinar A, Aydin H, Poyraz O. Influence of smoking on interleukin-1beta level, oxidant condition and antioxidant status in gingival crevicular fluid from chronic periodontitis patients before and subsequently periodontal treatment. J Periodontal Res. 2012;47:572–7.
-
Tuter G, Kurtis B, Serdar Grand, Aykan T, Okyay Thou, Yucel A, et al. Effects of scaling and root planing and sub-antimicrobial dose doxycycline on oral and systemic biomarkers of illness in patients with both chronic periodontitis and coronary artery disease. J Clin Periodontol. 2007;34:673–81.
-
Vardar S, Baylas H, Huseyinov A. Furnishings of selective cyclooxygenase-2 inhibition on gingival tissue levels of prostaglandin E2 and prostaglandin F2alpha and clinical parameters of chronic periodontitis. J Periodontol. 2003;74:57–63.
-
Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, Dalli J, et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J Paring Res. 2015;94:148–56.
-
Vyas SP, Sihorkar 5, Mishra V. Controlled and targeted drug commitment strategies towards intraperiodontal pocket diseases. J Clin Pharm Ther. 2000;25:21–42.
-
Wahid A, Chaudhry S, Ehsan A, Butt S, Khan AA. Bidirectional relationship between chronic kidney disease & periodontal disease. Pak J Med Sci. 2013;29:211–5.
-
Wang XE, Xu L, Meng HX, Lü D, Chen ZB, Lu RF. Long-term clinical and hematologic effects of non-surgical treatment on aggressive periodontitis. Zhonghua Kou Qiang Yi Xue Za Zhi. 2013;48:467–71.
-
Wehmeyer MM, Kshirsagar AV, Barros SP, Beck JD, Moss KL, Preisser JS, et al. A randomized controlled trial of intensive periodontal therapy on metabolic and inflammatory markers in patients With ESRD: results of an exploratory study. Am J Kidney Dis. 2013;61:450–viii.
-
Wei D, Zhang XL, Wang YZ, Yang CX, Chen G. Lipid peroxidation levels, total oxidant status and superoxide dismutase in serum, saliva and gingival crevicular fluid in chronic periodontitis patients earlier and later periodontal therapy. Aust Paring J. 2010;55:70–8.
-
Williams KB. Periodontal disease and blazon 2 diabetes. J Dent Hyg. 2009;83:eight–44.
-
Williams KB, Bray KK. Increasing patient appointment in care: motivational i nterviewing. Access. 2009;23:36–9.
-
Forest N. Oral health -- how to reduce risks of periodontitis. Posit Health. 2006:thirty-35.
-
Wu Y, Chen Fifty, Wei B, Luo K, Yan FH. Effect of Non-Surgical Periodontal Treatment on Visfatin Concentrations in Serum and Gingival Crevicular Fluid of Patients With Chronic Periodontitis and Blazon 2 Diabetes Mellitus. J Periodontol. 2015;86:795–800.
-
Zare Javid A, Seal CJ, Heasman P, Moynihan PJ. Impact of a customised dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. J Hum Nutr Diet. 2014;27:523–32. 510p.
-
Zhou SY, Duan XQ, Hu R, Ouyang XY. Effect of non-surgical periodontal therapy on serum levels of TNF-a, IL-6 and C-reactive protein in periodontitis subjects with stable coronary heart disease. Chin J Dent Res. 2013;16:145–51.
-
Zuza EP, Barroso EM, Carrareto AL, Pires JR, Carlos IZ, Theodoro LH, et al. The function of obesity every bit a modifying factor in patients undergoing not-surgical periodontal therapy. J Periodontol. 2011;82:676–82.
-
Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000. 2004;34:nine–21.
-
Silness J, Löe H. Periodontal Disease in Pregnancy Two. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35.
-
Löe HSJ. Periodontal disease in pregnancy. I. Prevalance and severity. Acta Odontol Scand. 1963;21:533–51.
Acknowledgment
The authors would similar to thank Mrs. Dr. Martina Gosteli, librarian of the main library of the Academy of Zurich who performed the electric literature search.
This study was supported by the Clinic of Preventive Dentistry, Periodontology and Cariology (Center of Dental Medicine) of the University of Zurich.
Authors' contributions
FAG and PRS conceived the study, participated in its design, did the literature search and drafted the manuscript. PS helped to supervise the methodological correctness of the performed study and the coordination. OAS and JHB provided the required medical theoretical background for this report and participated in the written report design. CH helped with the statistical evaluation of the papers and the tables. All authors carefully read and approved the concluding text.
Competing involvement
The authors declare that they take no competing interests.
Writer information
Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution iv.0 International License (http://creativecommons.org/licenses/by/four.0/), which permits unrestricted use, distribution, and reproduction in whatsoever medium, provided you lot give appropriate credit to the original writer(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the information made available in this commodity, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Gerber, F.A., Sahrmann, P., Schmidlin, O.A. et al. Influence of obesity on the result of non-surgical periodontal therapy - a systematic review. BMC Oral Health 16, 90 (2016). https://doi.org/ten.1186/s12903-016-0272-2
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12903-016-0272-ii
Keywords
- Obesity
- Chronic periodontitis
- Non-surgical periodontal therapy
- Outcome
Source: https://bmcoralhealth.biomedcentral.com/articles/10.1186/s12903-016-0272-2
0 Response to "Systematic Review Wit Hmeta Anlysis on Obesity and Periodontal Disease"
Enviar um comentário